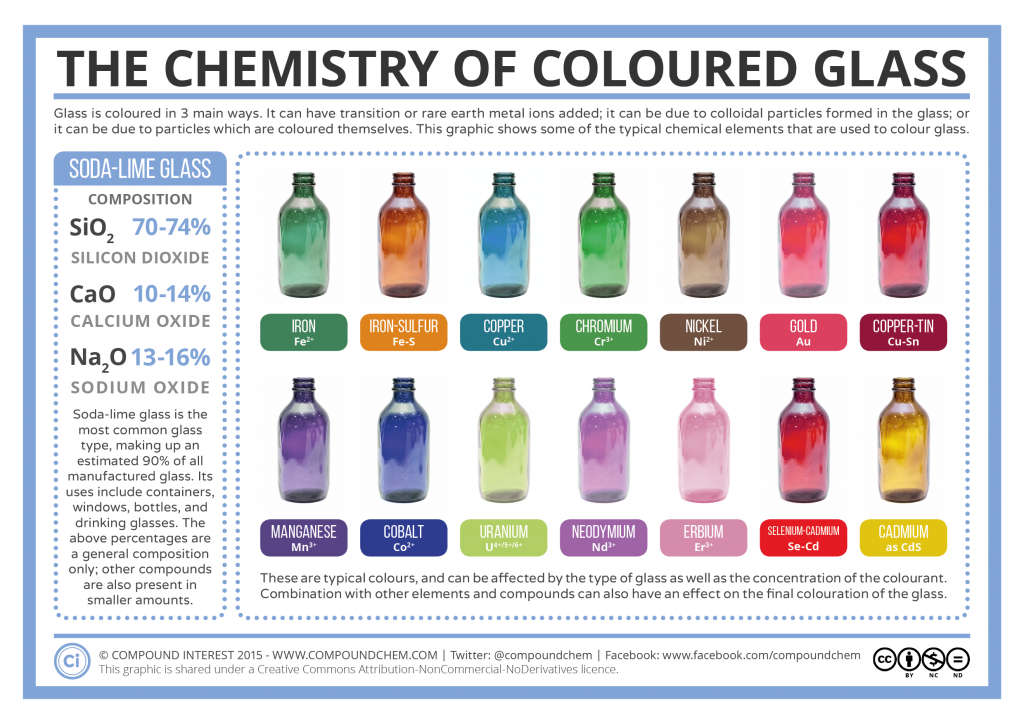
Soda Lime Glass is the most common glass type, making up an estimated 90% of all manufactured glass. Its uses include containers, windows, bottles and drinking glasses. The below percentages are a general composition only; other compounds are also present in smaller amount.
Silicon Dioxide (SiO2): 70-74%
Calcium Oxide (CaO): 10-14%
Sodium Oxide (Na2O): 13-16%
Glass is colored in three main ways. It can have a transition or rare earth metal ions added; it can be due to colloidal particles formed in the glass, or it can be due particles which are colored themselves.
There are several ways to impart color into glass. Most color is created by mixing a specific oxide into the batch and allowing it to react with the other constituents during the melting process. The results depend on a good number of variables, some of which you have control over and some of which you don’t. It is not surprising that only a small percentage of glassworkers make their own colors. Batching and melting color takes time, money, space, and extensive knowledge of glass chemistry. There are some serious health risks to think about as well.
Most glassblowers today use a pre-manufactured form of concentrated color that is compatible with the clear glass that they are melting in their furnace. These “pigments” are specifically formulated for applications in hot glass. The colors come in every color of the rainbow, either are transparent or opaque. Color is manufactured into rod, frit and powdered forms, and can be applied in a million different ways (or more). That’s the fun part.
Cobalt (CoCO3) – a very small addition of cobalt carbonate will turn your melt a deep dark blue, thus creating the all-time best selling color for glass blowers, cobalt blue. Other blues can be achieved with copper.
Chromium (Cr2O3) – adding this to the melt will yield an emerald green color
Copper (CuCO3) – Copper is one of those freaky chemicals that react quite differently with the other constituents of the melt. It is also highly susceptible to the atmospheric conditions of the melting chamber. So, depending on how you melt it, and with what, you can obtain: blues, greens, and even some tasty ruby reds (or if you’re off by a fraction, a nauseous liver-brown).
Manganese (MnO2) – chemists refer to this as a fugitive colorant. It gives rise to: purples, blue/violets, and some browns. Can also be affected by sunlight and U/V.
Silver (AgNO3) – can yield a variety of colors, from yellows to blues, and a wild mix of others depending on how you introduce it to the melt.
Gold (AuCl3) – the most beautiful ruby-red you may ever see. (a.k.a “granny-grabber pink” for its inherent ability to attract a certain member of our society) Gold must be introduced in a chloride form, and it too is very tricky to melt.
Iron (Fe2O3) – greens and browns Cadmium Sulfide (CdS) – oranges, also a challenging color to melt.
Cadmium selenium (CdSe) – deep ruby reds. Another tricky color to melt, in that the right temperature and atmosphere must be present in the furnace otherwise it will turn livery/brown.
Below is a graphic from compound interest shows some of the typical chemical elements that are used to color glass.
- Iron (Fe2+)
- Iron-Sulfur (Fe-S)
- Copper (Cu2+)
- Chromium (Cr3+)
- Nickel (Ni2+)
- Gold (Au)
- Copper-Tin (Cu-Sn)
- Manganese (Mn3+)
- Cobalt (Co2+)
- Uranium (U4+/5+/6+)
- Neodymium (Nd3+)
- Erbium (Er3+)
- Selenium-Cadmium (Se-Cd)
- Cadmium (as CdS)
These are typical colors, and can be affected by the type of glass, as well as the concentration of the colorant. Combination with other elements and compounds can also have an effect on the final coloration of the glass.
![Inorganic Compounds As Pigments in Paints [Infographic] Inorganic Compounds As Pigments in Paints](https://chemistry.com.pk/wp-content/uploads/2014/08/Inorganic-Compounds-As-Pigments-in-Paints.png)
![Colours of Transition Metal Ions in Aqueous Solution [Infographic] Colours of Transition Metal Ions](https://chemistry.com.pk/wp-content/uploads/2014/08/Colours-of-Transition-Metal-Ions.png)
![Testing for Cations By Sodium Hydroxide & Ammonia Precipitates [Infographic] Metal Ion Precipitates](https://chemistry.com.pk/wp-content/uploads/2014/08/Metal-Ion-Precipitates.jpg)