Virtually all organic reactions fall into one of four categories: substitutions, additions, eliminations, or rearrangements.
Substitution Reactions
Substitutions are the characteristic reactions of saturated compounds such as alkanes and alkyl halides and of aromatic compounds (even though they are unsaturated). In a substitution, one group replaces another. For example, chloromethane reacts with sodium hydroxide to produce methyl alcohol and sodium chloride:
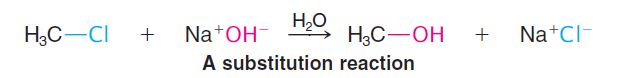
In this reaction a hydroxide ion from sodium hydroxide replaces the chlorine of methyl chloride.
Addition Reactions
Additions are characteristic of compounds with multiple bonds. Ethene, for example, reacts with bromine by an addition. In an addition all parts of the adding reagent appear in the product; two molecules become one:
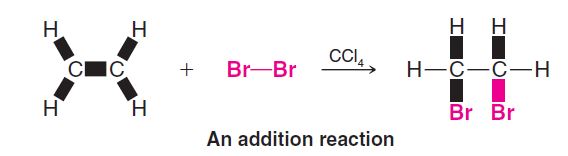
Elimination Reactions
Eliminations are the opposite of additions. In an elimination one molecule loses the elements of another small molecule. Elimination reactions give us a method for preparing compounds with double and triple bonds.
There is an important an elimination called dehydrohalogenation, a reaction that is used to prepare alkenes. In dehydrohalogenation, as the word suggests, the elements of a hydrogen halide are eliminated. An alkyl halide becomes an alkene:
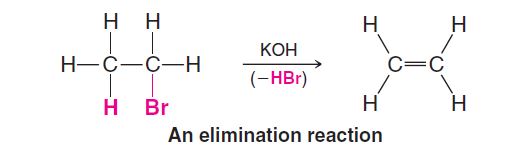
Rearrangements
In a rearrangement a molecule undergoes a reorganization of its constituent parts. For example, heating the following alkene with a strong acid causes the formation of another isomeric alkene:
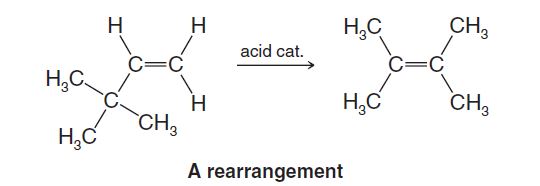
In this rearrangement not only have the positions of the double bond and a hydrogen atom changed, but a methyl group has moved from one carbon to another.