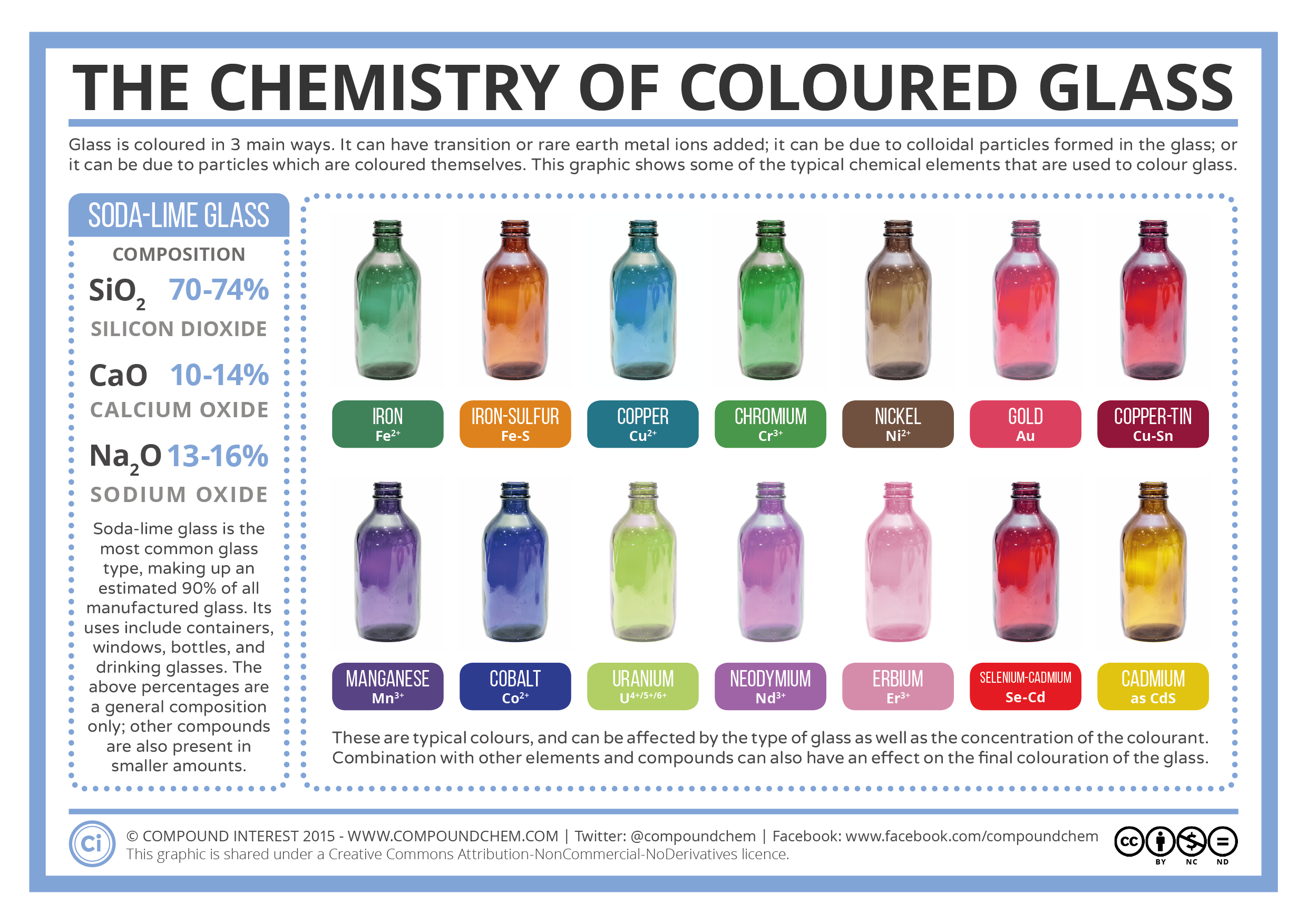
A guide to the different ways Organic Compounds can be represented in chemistry. Types of Organic Chemistry Formulae discussed in this infographic are:
Molecular Formula
The molecular formula of an organic compound simply shows the number of each type of atom present. It tells you nothing about the bonding within the compound.
Empirical Formula
The empirical formula of an organic compound gives the simplest possible whole number ratio of the different types of the atom within the compound.
Condensed Formula
A displayed formula shows all the atoms and all of the bonds present in an organic compound. The bonds are presented as lines.
Structural Formula
Similar to displayed formula – not all bonds are shown, although all atoms are still indicated using subscript numbers. Carbon hydrogen bonds are often simplified.
Skeletal Formula
In a skeletal formula most hydrogen atoms are omitted, and line ends or vertices represents carbons. Functional groups and atoms other than carbon or hydrogen are still shown. Easiest to draw and commonly used.
Read full article on A Brief Guide to Types of Organic Chemistry Formulae and download pdf. of this infographic on Compound Interest.
![Inorganic Compounds As Pigments in Paints [Infographic] Inorganic Compounds As Pigments in Paints](https://chemistry.com.pk/wp-content/uploads/2014/08/Inorganic-Compounds-As-Pigments-in-Paints.png)