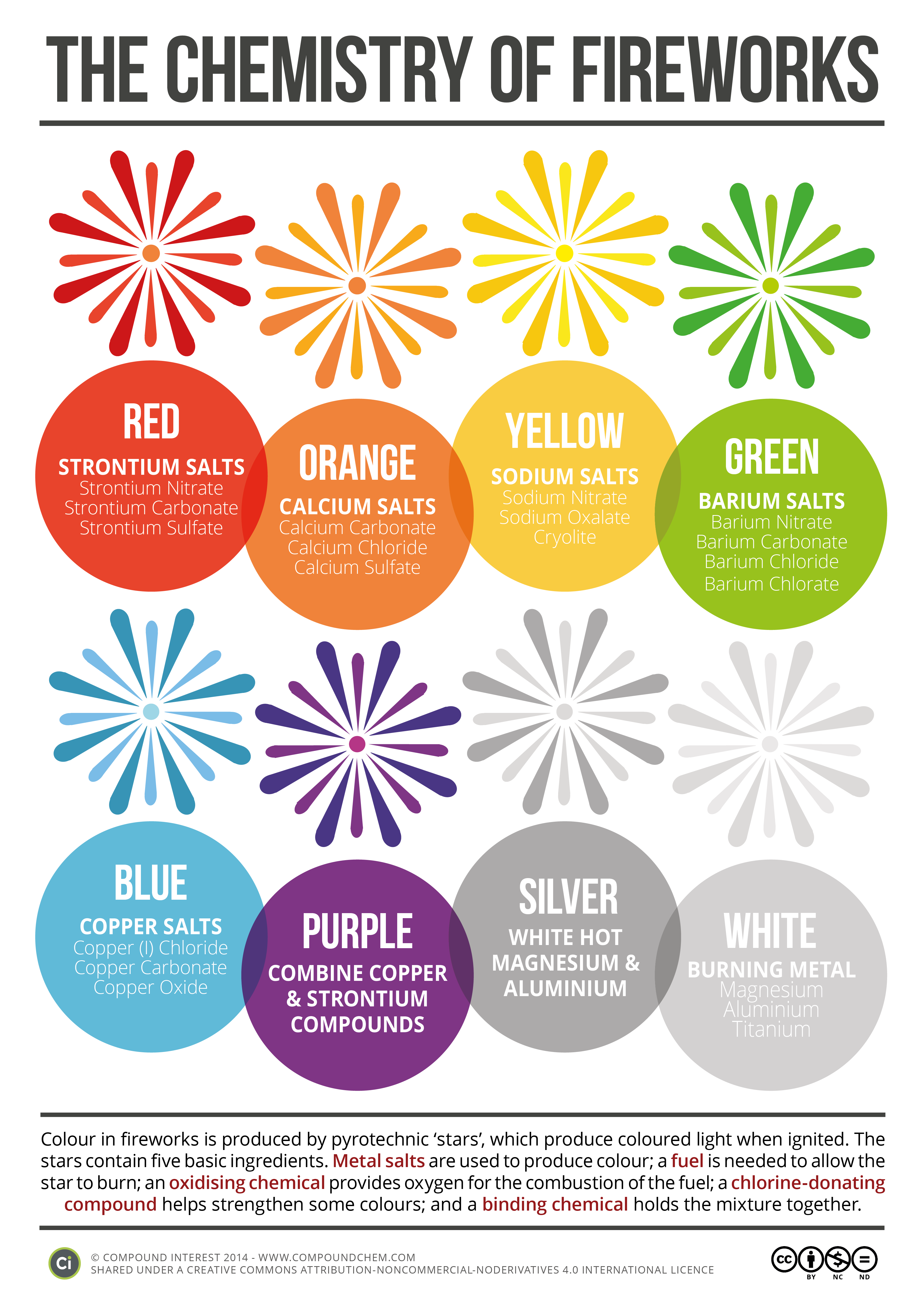
Colour in fireworks is produced by pyrotechnic ‘stars’, which produce coloured light when ignited. The stars contain five basic ingredients. Metal salts are used to produced colour; a fuel is needed to allow the star to burn; an oxidising chemical provides oxygen for the combustion the fuel; a chlorine-donating compound helps strengthen some colours; and a binding chemical holds the mixture together.
The following fireworks colour described in this infographic.
- Red
- Orange
- Yellow
- Green
- Blue
- Purple
- Silver and
- White
Click on Image to Enlarge
Read full article on The Chemistry of Fireworks and download pdf. of this infographic on Compound Interest.
![Inorganic Compounds As Pigments in Paints [Infographic] Inorganic Compounds As Pigments in Paints](https://chemistry.com.pk/wp-content/uploads/2014/08/Inorganic-Compounds-As-Pigments-in-Paints.png)
![Colours of Transition Metal Ions in Aqueous Solution [Infographic] Colours of Transition Metal Ions](https://chemistry.com.pk/wp-content/uploads/2014/08/Colours-of-Transition-Metal-Ions.png)
![Metal Ion Flame Test Colours [Infographic] Metal Ion Flame Test](https://chemistry.com.pk/wp-content/uploads/2014/08/Metal-Ion-Flame-Test.jpg)